A closed tank is partially filled with glycerin, an intriguing liquid with unique physical properties. This analysis delves into the behavior of glycerin within the confines of a closed tank, examining the interplay between pressure, volume, and temperature.
Glycerin’s density, viscosity, and specific heat capacity are fundamental properties that significantly influence its behavior in the tank. These properties dictate how glycerin responds to changes in pressure, volume, and temperature, shaping its overall characteristics.
Closed Tank Analysis: Glycerin Behavior: A Closed Tank Is Partially Filled With Glycerin
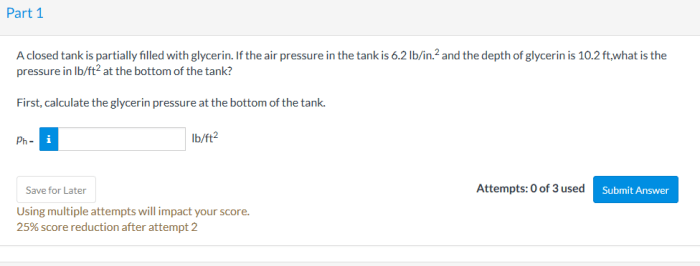
A closed tank partially filled with glycerin presents unique characteristics due to the liquid’s distinct physical properties. This analysis explores the behavior of glycerin in a closed tank, examining pressure-volume relationships, temperature effects, and real-world applications.
Physical Properties of Glycerin
Glycerin, a viscous, colorless liquid, exhibits several key physical properties that influence its behavior in a closed tank:
- Density:Glycerin has a relatively high density, contributing to its weight and pressure distribution.
- Viscosity:Glycerin’s high viscosity creates resistance to flow, affecting its movement within the tank.
- Specific Heat Capacity:Glycerin’s high specific heat capacity indicates its ability to absorb and release heat without significant temperature changes.
Pressure and Volume Relationships
In a closed tank, the pressure exerted by glycerin on its surroundings is influenced by its volume. The relationship between pressure and volume follows Boyle’s Law:
P1V 1= P 2V 2
where P represents pressure, V represents volume, and subscripts 1 and 2 denote initial and final conditions.
Gauge pressure, which measures pressure relative to atmospheric pressure, is often used in closed tank analysis. Absolute pressure, on the other hand, measures pressure relative to a perfect vacuum.
Temperature Effects, A closed tank is partially filled with glycerin
Temperature significantly affects glycerin’s properties and the pressure-volume relationship in a closed tank. As temperature increases:
- Density:Glycerin’s density decreases, reducing its weight and pressure.
- Viscosity:Glycerin’s viscosity decreases, making it flow more easily.
- Specific Heat Capacity:Glycerin’s specific heat capacity remains relatively constant.
These temperature-induced changes impact the pressure-volume relationship and can alter the behavior of glycerin in the tank.
Applications and Examples
Understanding the behavior of glycerin in a closed tank has practical applications in various industries:
- Hydraulic Systems:Glycerin’s high viscosity and lubricity make it suitable for hydraulic systems, where it transmits pressure and power.
- Food and Beverage Industry:Glycerin is used as a sweetener and humectant in food and beverages due to its high specific heat capacity.
- Cosmetics:Glycerin’s moisturizing properties make it a common ingredient in skincare and hair care products.
Question Bank
What is the significance of glycerin’s viscosity in a closed tank?
Glycerin’s high viscosity imparts resistance to flow, affecting the rate at which pressure changes propagate through the liquid. This property is crucial in applications involving hydraulic systems and damping mechanisms.
How does temperature influence the pressure-volume relationship in the tank?
Temperature changes alter the density and viscosity of glycerin, thereby affecting the pressure-volume relationship. As temperature increases, the density decreases, leading to a decrease in pressure for a given volume. Conversely, an increase in temperature reduces viscosity, facilitating easier flow and pressure equalization.