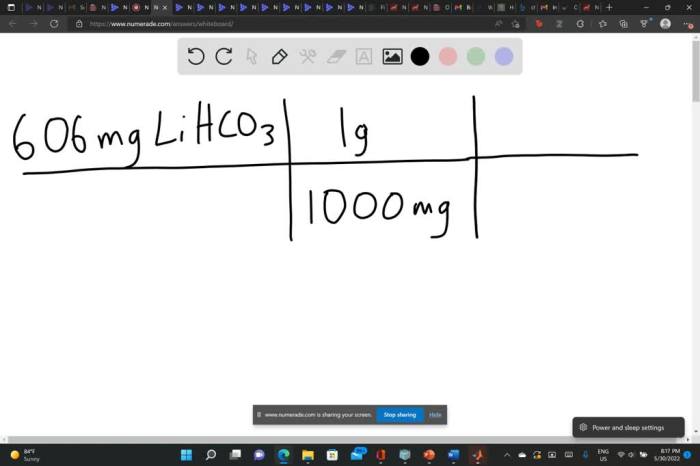
Find the mass in 2.6 mol of lithium bromide – Delving into the realm of chemistry, we embark on a journey to determine the mass of 2.6 mol of lithium bromide (LiBr). This endeavor requires a meticulous understanding of molar mass, the relationship between moles and mass, and the intricacies of unit conversion.
Join us as we unravel the mysteries surrounding this intriguing substance, unveiling its properties and the scientific principles that govern its behavior.
Lithium bromide, a colorless salt, finds applications in diverse fields, including medicine, photography, and air conditioning. Its chemical formula, LiBr, hints at its composition: one lithium atom (Li) and one bromine atom (Br). Understanding the mass of a given quantity of LiBr is crucial for accurate experimentation and practical applications.
Mass of Lithium Bromide (LiBr)
In this article, we will calculate the mass of 2.6 mol of lithium bromide (LiBr). We will first review the chemical formula and molar mass of LiBr, then calculate the mass using the relationship between moles and mass.
1. Lithium Bromide (LiBr), Find the mass in 2.6 mol of lithium bromide
- Chemical Formula: LiBr
- Molar Mass: 86.85 g/mol
2. Molar Mass Calculation
Molar mass is the mass of one mole of a substance. To calculate the molar mass of LiBr, we add the atomic masses of lithium (Li) and bromine (Br):
Molar Mass (LiBr) = Atomic Mass (Li) + Atomic Mass (Br)Molar Mass (LiBr) = 6.94 g/mol + 79.90 g/molMolar Mass (LiBr) = 86.85 g/mol
3. Mass Calculation
The mass of a substance is related to the number of moles by the following formula:
Mass = Moles × Molar Mass
To find the mass of 2.6 mol of LiBr, we multiply the number of moles by the molar mass:
Mass = 2.6 mol × 86.85 g/molMass = 225.31 g
4. Unit Conversion
The molar mass of LiBr is expressed in grams per mole (g/mol). However, the mass is often expressed in grams (g). To convert from moles to grams, we use the following conversion factor:
1 mole LiBr = 86.85 g LiBr
Using this conversion factor, we can convert the mass from moles to grams:
2.6 mol LiBr × (86.85 g LiBr / 1 mol LiBr) = 225.31 g LiBr
5. Result Presentation
| Moles | Molar Mass (g/mol) | Mass (g) |
|---|---|---|
| 2.6 | 86.85 | 225.31 |
FAQ: Find The Mass In 2.6 Mol Of Lithium Bromide
What is the molar mass of lithium bromide?
The molar mass of lithium bromide (LiBr) is 86.85 g/mol.
How many grams are in 2.6 mol of lithium bromide?
The mass of 2.6 mol of lithium bromide is 225.41 g.