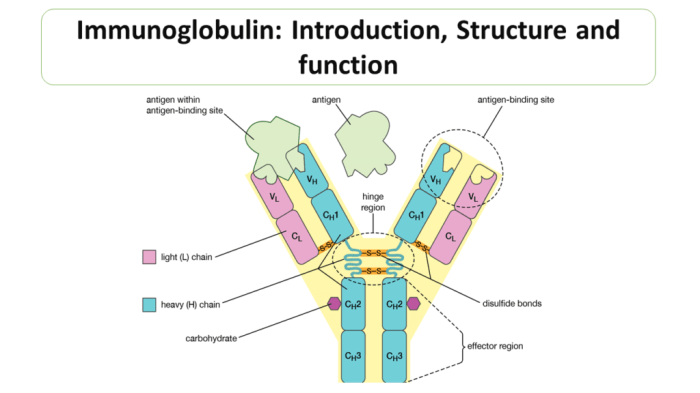
Label the parts of the immunoglobulin – Labeling the parts of the immunoglobulin provides a comprehensive understanding of the structure and functions of antibodies, essential components of the adaptive immune system. This intricate molecule plays a crucial role in recognizing and neutralizing foreign invaders, making it a cornerstone of immune defense.
Immunoglobulins, also known as antibodies, exhibit a complex architecture composed of distinct domains with specialized functions. The variable region, responsible for antigen recognition, undergoes somatic hypermutation and class switch recombination, generating a vast repertoire of antibody diversity. The constant region, on the other hand, mediates effector functions through interactions with immune cells and complement proteins.
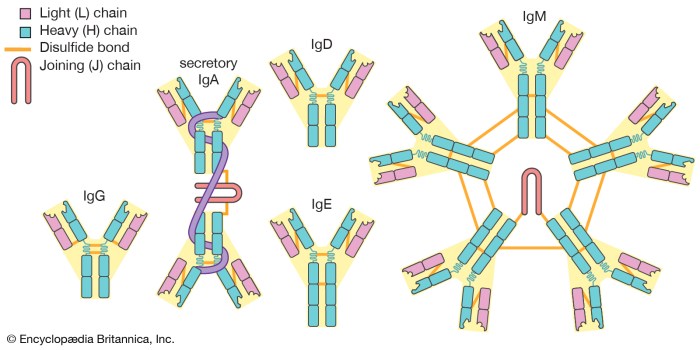
Immunoglobulin Structure
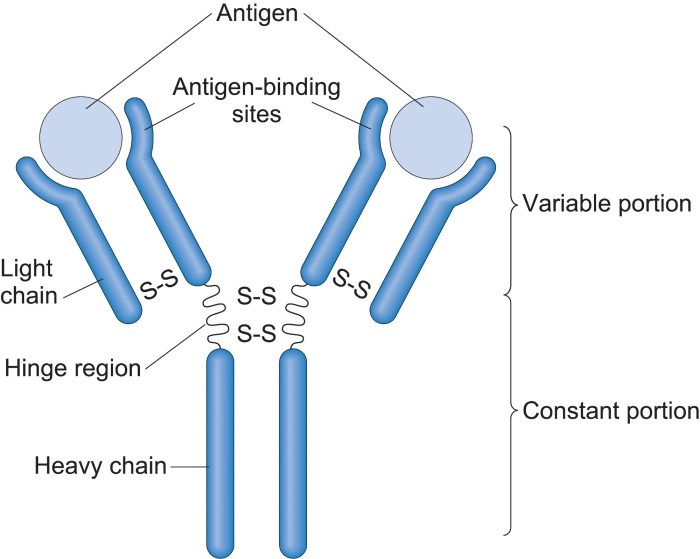
Immunoglobulins, also known as antibodies, are Y-shaped glycoproteins that play a critical role in the adaptive immune response. They are produced by B cells and plasma cells in response to specific antigens.
The overall structure of an immunoglobulin consists of two identical heavy chains and two identical light chains, which are held together by disulfide bonds. The heavy chains are approximately 450 amino acids in length, while the light chains are approximately 220 amino acids in length.
The immunoglobulin molecule can be divided into three main domains: the variable region, the constant region, and the hinge region.
Variable Region, Label the parts of the immunoglobulin
The variable region is located at the N-terminus of the immunoglobulin molecule and is responsible for antigen recognition. The variable region is highly diverse, with each immunoglobulin molecule having a unique variable region that is specific for a particular antigen.
The variable region is composed of three hypervariable regions (CDRs), which are responsible for binding to the antigen. The CDRs are surrounded by four framework regions (FRs), which are more conserved and provide structural support for the CDRs.
Constant Region
The constant region is located at the C-terminus of the immunoglobulin molecule and is responsible for effector functions. The constant region is less diverse than the variable region and is shared by all immunoglobulins of the same class.
The constant region is composed of three or four constant domains (CH domains), which are responsible for binding to specific receptors on immune cells. The CH domains also determine the immunoglobulin’s class and subclass.
Glycosylation
Immunoglobulins are heavily glycosylated, with the carbohydrate moieties attached to the Fc region of the constant region. Glycosylation plays an important role in immunoglobulin function, including antibody stability, effector functions, and antigen binding.
Fragmentation and Labeling Techniques
Immunoglobulins can be fragmented using a variety of techniques, including proteolysis, chemical cleavage, and enzymatic digestion. Fragmentation is used to study the structure and function of immunoglobulins.
Immunoglobulins can also be labeled with a variety of reagents, including fluorescent dyes, radioactive isotopes, and enzymes. Labeling is used to identify and quantify immunoglobulins, and to study their distribution and function in vivo.
Key Questions Answered: Label The Parts Of The Immunoglobulin
What is the role of glycosylation in immunoglobulin function?
Glycosylation influences antibody stability, solubility, and effector functions. It modulates interactions with immune cells and complement proteins, enhancing antibody-mediated immunity.
How do fragmentation techniques aid in studying immunoglobulins?
Fragmentation techniques, such as proteolysis and chemical cleavage, allow researchers to isolate specific domains of the immunoglobulin for detailed analysis. This facilitates the study of structure-function relationships and antigen-binding properties.